What Does a Glacier Mummy Tell Us About Cryopreservation?
Even after five thousand years in ice, the proteins in Ötzi’s brain still tell a story of molecular survival, potentially holding unexpected lessons for the future of cryopreservation
In 1991, the frozen remains of a man who died over 5,000 years ago were recovered from the Ötztal Alps. Nicknamed Ötzi the Iceman, his preservation was so striking that some popular accounts likened it to cryonics. However, Ötzi was not cryopreserved; his body underwent slow freezing, partial desiccation, and ice crystallization; all these conditions guarantee cellular death. Nevertheless, modern analytical tools have revealed an extraordinary amount of molecular information from his tissues, including intact protein structures, glycans, and even red blood cell morphology (all non-functional, though).
This raises a provocative question: if we analyze which proteins survived and which degraded in naturally frozen mummies, do we gain insights relevant to modern cryopreservation science?
🔴 TRIGGER WARNING: I included the photo of Ötzi at the end of the article
“Hey Professor, we found a cryo-mummy” - what do you do?
Maixner et al.’s team must have been pretty shocked when they heard about Ötzi; it was an opportunity that they for sure have not anticipated to ever stumble upon. So, in such an unprecedented scenario, what did they do? First, they had to figure out what to look for. DNA could reveal where he came from and what traits he carried, but to understand what was happening in his body at the time of death, they needed to study the proteins. The team set out to reconstruct the proteome (the complete set of detectable proteins from the Iceman’s brain) to see if any molecular traces of stress or injury had survived.
But before they could extract anything, they had to decide where to sample. Earlier CT scans had shown a that only the occipital lobe of the brain (“vision region”) was relatively well preserved. Guided by CT imaging and 3D navigation, the researchers performed a precise endoscopic procedure to collect two tiny, rice-grain-sized, biopsies from this region. Because the tissue had been desiccated for more than five millennia, it was first rehydrated using saline to allow ancient proteins to dissolve again, and then treated with a solvent that breaks open cell membranes and unfolds proteins without destroying them, particularly effective for lipid-rich neural material. Two complementary extraction protocols were used: one gentle, targeting soluble proteins, and one harsher, involving longer sonication cycles to release material still bound within the debris. The final step was liquid chromatography–mass spectrometry (LC–MS) which eventually revealed over 500 proteins, including neuronal cytoskeletal proteins (neurofilament light and medium chains), synaptic vesicle proteins (synapsins), and glial fibrillary acidic protein (GFAP). These findings confirm that not only connective tissue but actual neuronal and glial structures persisted after 5,000 years.
The types and properties of the proteins that remained
So, what kind of proteins actually made it through five millennia of freezing, drying, and slow decay? And do they have anything in common like structure or function that makes them tougher than others?
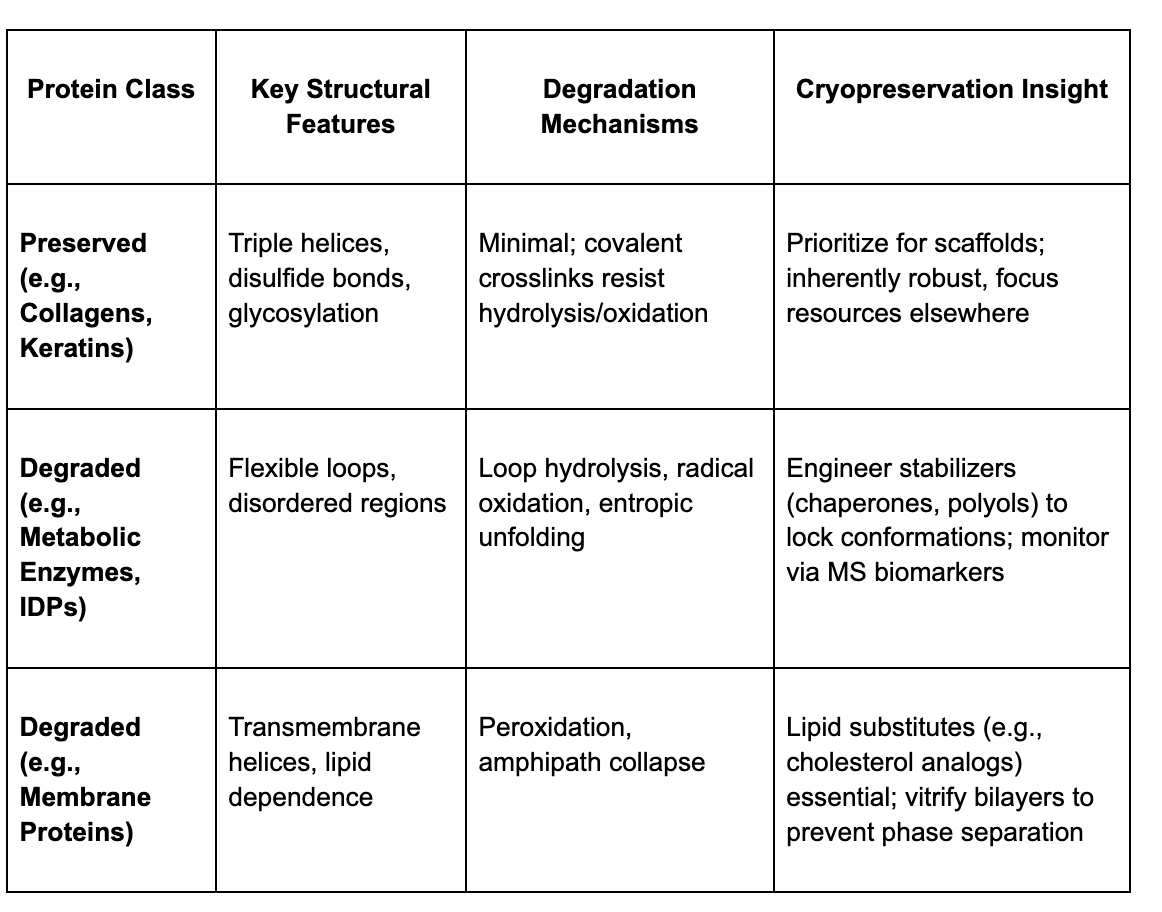
The survivors tend to be structural and extracellular proteins. Collagens (yes, the ones from your longevity powders), for example, have rope-like triple-helix structure, reinforced by special chemical bonds, giving them exceptional resistance to breakdown. Keratins, the proteins that form hair and skin, also endure thanks to tight internal bridges that protect them from oxidation and water damage. In the Iceman’s case, researchers also found blood-clotting and wound-related proteins, such as fibrin and fibronectin, in the region of the brain where CT scans had shown trauma. These proteins naturally form dense networks when blood coagulates, which may have helped them resist enzymatic decay. Small fragments of hemoglobin, trapped within the remains of red blood cells, also survived, protected by the cell’s lipid envelope and the stabilizing iron in the heme group. Another group of persistent molecules were glycoproteins decorated with sugar chains, or N-linked glycans. These sugars act like natural armor, shielding the underlying protein from chemical attack. Similar protective effects likely explain why immune proteins such as complement fragments and serpins have also been detected in other mummified tissues as their modular, compact shapes make them unusually stable.
By contrast, proteins that are flexible or metabolically active in life like enzymes or membrane-bound transporters were almost completely gone. These molecules rely on fluid, dynamic shapes that collapse once the cell dies and its lipid membranes disintegrate.
In short, the molecular survivors of deep time are those built for strength, not speed; proteins meant to provide scaffolding, protection, or repair, persisted.
Here is a quick table for comparison:
Structural Lessons
The Iceman’s proteome reinforces a key principle of cryopreservation: stability isn’t just about temperature, it’s about molecular architecture. What survived did so through dense crosslinking, restricted mobility, and built-in redundancy; what didn’t were molecules that relied on flexibility and fluidity for function.For cryobiology, that distinction is crucial. The same forces that destroyed Ötzi’s enzymes and membranes like dehydration, oxidation, and local pH shifts also threaten modern tissues during freezing and thawing. The lesson is that preservation must be structural as well as thermal. If we want to safeguard living systems, we need strategies that reinforce the vulnerable domains of proteins like stabilizing active sites, shielding flexible loops, and preventing lipid peroxidation before cooling ever begins.
The challenge for cryobiology is to reverse-engineer those outcomes deliberately: to design environments where even the dynamic and fragile parts of life can withstand the long pause, and still remember how to move when warmth returns.




I must say that this person's life mattered more than the lives of his peers as it gave us valuable information, not just compost
This is a great story. I used to sell collagen briefly without even realizing it had some of those effects. 🙏👏